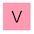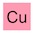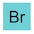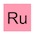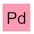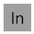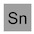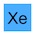I received the e-mail from @SeeArrOh a little while ago about the impending #ChemMovieCarnival, but I figured I would just be too busy to join in. After seeing the first round up of posts, however, I couldn’t resist, so here’s my contribution.
I’m going to tackle the cinematic masterpiece that is Superman III — there really is some fantastic chemistry in there that I want to share with you (you might want to look up all of the definitions of ‘fantastic’…).
Clark Kent (I’m not gonna spoil it for you guys if I tell you that he’s really Superman am I?) is heading back to Smallville to go to his high-school reunion when the bus he’s travelling on is pulled over because there is a fire in a chemical plant close to the road. The cop who pulls over the bus is perhaps exhibiting some mild symptoms of chemophobia when he tells the driver that, “It’s not just a building, it’s a chemical plant. You know what I mean, it’s like, err, it’s like chemicals.” It’s not long before Superman arrives on the scene and he sets about rescuing workers trapped on (and in) the building. In one room he finds a scientist (he must be a scientist, he’s wearing a lab coat) who refuses to leave — the conversation goes like this:
Scientist: I gotta stay and look after those. That’s concentrated beltric acid. If that stuff heats up over 180 degrees we’ve got a crisis on our hands that’ll make this fire look like a Sunday-school picnic.
Superman: What does it do?
Scientist: As long as it remains stable it’s just ordinary acid, no problem. But if it begins to heat up, it’ll turn volatile. If that happens you’ll get a great cloud of smoke that’ll eat through anything, steel, concrete, anything.

Oh my! It’s beltric acid. And that dial, all it seems to measure is ‘DANGER’. What are the SI units for DANGER?
Superman, of course, saves the day, and the beltric acid is safe. For now.
The movie continues, and the villain of the piece — businessman Ross Webster (played by Robert Vaughn) — decides to get rid of Superman after the man of steel thwarts his plans to destroy Colombia’s coffee crop. How do you get rid of Superman? Well, you just need some kryptonite. And what if you can’t get any kryptonite? Simple, just figure out what it is made of and then synthesize some in the lab — yay, chemical synthesis FTW!
After Gus Gorman (played by the genius Richard Pryor) gets caught skimming off the half cents not paid to the employees of Webscoe into his own expenses account, Webster puts Gorman’s computer programming skills to use in his evil schemes — including the plan to make kyrptonite. So, how does it all work? If you haven’t already suspended belief yet, now would be good. Gorman hacks into a weather satellite and uses it scan the region of space where the planet Krypton used to be. It amuses me that the computer can’t spell…

‘i’ before ‘e’, except after ‘c’ when… you know the rest.
Webster describes the rest of the plan in a voice-over:
Then the laser probe simply locks on to a floating chunk of kryptonite, the computer analyses the components, and the boys at the lab duplicate the stuff down here.
‘Simply’?! OK, first off, I know this is a movie, but allow me to point out the flaw in the logic here. You don’t know what kryptonite is, so how do you lock on to a floating chunk of it? How do you know that you haven’t locked on to lump of adamantium, dilithium or vibranium? Anyway, back to the plot. That’s some awesome analytical chemistry going on right there. A laser fired from a satellite hits a lump of kryptonite and the computer back on Earth figures out exactly what it’s made of — here are the results:

Decrypting kryptonite. Dialium anyone? And no krypton? That’s disappointing.
That’s some pretty potent stuff right there. I’m not from Krypton, but you wouldn’t catch me going anywhere near Kryptonite.
Rather than leaving it to the scientists to decide what to do about that small amount of ‘unknown’ — such as just leaving it out — Gorman gets some inspiration from the side of his cigarette packet and decides to swap ‘unknown’ for ‘tar’. The details get sent off to the ‘boys at the lab’ and they set about making synthetic kryptonite — it must have been a fun prep… and just imagine filling in the safety assessment for that one! (They do full safety assessments in the labs housed in the lairs of evil geniuses don’t they?) And look, they even managed to crystallize the product:

PuTaXePmDa(?)HgC — or synthetic kryptonite if you prefer.
The whole synthetic-kryptonite plot-line is discussed in more detail at this website — I’ve tried to avoid directly repeating what is said over there and I recommend that you go and have a read to learn about alternative theories on the composition of kryptonite, including the one put forward in Superman Returns and its similarity to the naturally occurring Earth mineral jadarite. If you really want to delve deeper, apparently there are lots of different forms of kryptonite (polymorphs perhaps?).
To finish off, however, let’s bring this back down to Earth. Did you know that ‘kryptonite’ has been synthesized in a real chemistry lab and the results were reported in JACS? Yes, really! The paper, Isolation and Spectral Properties of Kr@C60, a Stable van der Waals Molecule, was published in 1999 and the kryptonite in question is the compound made up of a krypton atom trapped inside the buckminsterfullerene cage — an example of an endohedral fullerene. Seems like a reasonable name to me, it has krypton in it after all! And here’s the proof from the paper itself:

JACS — the journal of choice for all of your synthetic kryptonite work.
Alas, a search for ‘beltric acid’ in the scientific literature didn’t turn anything up. So, chemists of the world, here’s your challenge. Who will be the first to make a new compound called beltric acid and get it published in a reputable chemistry journal? That would be super, man.

![[Images taken from the websites of the respective publishers and copyright NPG, Wiley, ACS, RSC, respectively]](https://stuartcantrill.com/wp-content/uploads/2013/06/journals.jpg?w=600&h=150)